April 1, 2016
Impact of CMS Competitive Bidding Program on Medicare Beneficiary Safety and Access to Diabetes Testing Supplies: A Retrospective, Longitudinal Analysis
OBJECTIVE
In 2011, the Centers for Medicare & Medicaid Services (CMS) launched the Competitive Bidding Program (CBP) in nine markets for diabetes supplies. The intent was to lower costs to consumers. Medicare claims data (2009–2012) were used to confirm the CMS report (2012) that there were no disruptions in acquisition caused by CBP and no changes in health outcomes.
RESEARCH DESIGN AND METHODS
The study population consisted of insulin users: 43,939 beneficiaries in the nine test markets (TEST) and 485,688 beneficiaries in the nontest markets (NONTEST). TEST and NONTEST were subdivided: those with full self-monitoring of blood glucose (SMBG) supply acquisition (full SMBG) according to prescription and those with partial/no acquisition (partial/no SMBG). Propensity score–matched analysis was performed to reduce selection bias. Outcomes were impact of partial/no SMBG acquisition on mortality, inpatient admissions, and inpatient costs.
RESULTS
Survival was negatively associated with partial/no SMBG acquisition in both cohorts (P < 0.0001). Coterminous with CBP (2010–2011), there was a 23.0% (P < 0.0001) increase in partial/no SMBG acquisition in TEST vs. 1.7% (P = 0.0002) in NONTEST. Propensity score–matched analysis showed beneficiary migration from full to partial/no SMBG acquisition in 2011 (1,163 TEST vs. 605 NONTEST) was associated with more deaths within the TEST cohort (102 vs. 60), with higher inpatient hospital admissions and associated costs.
CONCLUSIONS
SMBG supply acquisition was disrupted in the TEST population, leading to increased migration to partial/no SMBG acquisition with associated increases in mortality, inpatient admissions, and costs. Based on our findings, more effective monitoring protocols are needed to protect beneficiary safety.
Introduction
Self-monitoring of blood glucose (SMBG) is a critical component of diabetes care among individuals treated with insulin (1,2). The American Diabetes Association recommends that all insulin-treated individuals should perform SMBG according to the needs of their insulin regimen (1). For individuals treated with intensive insulin regimens, SMBG should be performed at least prior to meals/snacks and occasionally postprandially, at bedtime, prior to exercise, when a low blood glucose is suspected, after treating low blood glucose, and prior to critical tasks such as driving; however, the evidence is insufficient regarding SMBG frequency in individuals treated with basal insulin therapy (1).
The American Association of Clinical Endocrinologists and American College of Endocrinology recommend that SMBG be performed by all patients using insulin (minimum of twice daily and ideally before any insulin injection) (2). More frequent SMBG after meals or in the middle of the night may be required for insulin-taking patients with frequent hypoglycemia, patients not at HbA1c targets, and those with hypoglycemic symptoms (2).
Frequent SMBG is particularly important in elderly individuals with diabetes who are treated with insulinotropic medications because the risk of severe or fatal hypoglycemia associated with the use of sulfonylureas or insulin increases exponentially with age (3,4). A study by Huang et al. (5) found that among individuals aged 70–79 years with a long duration of diabetes, incidence of hypoglycemia (15.88 per 1,000 person-years) was significantly higher than incidence of other complications.
Other factors that put elderly individuals with diabetes at significant risk for severe hypoglycemia are hypoglycemia unawareness (6) and cognitive impairment (7). A study by Bremer et al. (6) reported that patients aged ≥65 years with type 2 diabetes often fail to perceive neuroglycopenic and autonomic hypoglycemic symptoms even in the presence of a comparable prolongation of reaction time induced by hypoglycemia. It is also known that elderly individuals with diabetes (>65 years) are at increased risks of cognitive impairment and dementia (8), which are significantly associated with subsequent episodes of severe hypoglycemia (7).
It was recently reported that insulin-treated patients with diabetes 80 years or older were more than twice as likely to visit the emergency department and nearly five times as likely to be subsequently hospitalized for insulin-related hypoglycemic events than those aged 45–64 years (9). Although investigators cited missed/inadequate meals and insulin product mix-ups as the most common precipitating factors documented for these events (9), hypoglycemia unawareness (6) and/or cognitive impairment (7) may have been missed by emergency care personnel as the root causes of the severe hypoglycemia reported, which illustrates the need for frequent SMBG within this population.
Frequent use of SMBG in all insulin-treated patients can help mitigate hypoglycemia. However, the challenges associated with financing and providing access to diabetes testing supplies to patients with diabetes who are ≥65 years old are likely to grow with the increasing incidence of diabetes in the U.S. and the increased number of patients in this age-group.
To address the increasing financial burden of diabetes care among older Americans, the Centers for Medicare & Medicaid Services (CMS) implemented its Competitive Bidding Program (CBP) in nine test markets that included 2.3 million beneficiaries in Fee-for-Service Medicare (10). The intent of the CBP is to reduce beneficiary out-of-pocket expenses and reduce Medicare costs while ensuring beneficiary access to quality items and services (10).
SMBG supplies were among the products included in the program. Although not reported by CMS, it is our understanding that the agency selected the nine test markets for SMBG supplies based on population size. The assumed intent was to select markets that represented 10% of the SMBG supply volume covered by Fee-for-Service Medicare. However, only SMBG supplies obtained through mail-order channels were impacted in the initial implementation; single payment rates were reduced from $34 to $14 per vial of test strips. SMBG supplies obtained through retail channels were exempted from the first round of the program.
In April 2012, CMS reported that no disruption of access to diabetes testing supplies occurred and that no negative health care consequences to beneficiaries were seen as a result of the CBP (10). Recognizing the potential benefits of reducing out-of-pocket expenses for SMBG supplies among Medicare beneficiaries, particularly within minority populations, the National Minority Quality Forum obtained data from CMS to more fully elucidate the effects of the implementation of the CBP on acquisition of SMBG supplies among Medicare beneficiaries with insulin-treated diabetes within the nine test markets. We hypothesized that lower SMBG costs would encourage greater SMBG supply acquisition, resulting in fewer hospitalizations and lower inpatient costs.
Research Design and Methods
In this 4-year, retrospective, longitudinal study, we assessed the impact of competitive bidding during the first year of program implementation among Medicare beneficiaries who treated their diabetes using insulin within the nine test markets (TEST) compared with insulin-using beneficiaries in the nontest markets (NONTEST), which represent the rest of the country. For our analysis, we obtained the same data collected by CMS to assess impact and outcomes. Our goal was to determine whether access to SMBG supplies improved in the year after CBP implementation (2011) and, if so, assess the behavioral and health outcomes resulting from access improvement.
Access was assessed according to each beneficiary’s acquisition of insulin and SMBG supplies as prescribed by their health care provider. For a beneficiary on insulin, Medicare reimburses for the acquisition of three strips per day. Based on that reimbursement schedule, full procurement of self-monitoring blood glucose supplies is defined here as the purchase of diabetes testing strips so that, from the date of the first purchase, the beneficiary continued to acquire testing supplies, resulting in their purchasing enough blood glucose testing supplies to allow them to test their blood glucose three times per day >80% of the year. Any beneficiary who scored 80% or higher on this proportional days–covered (PDC) scale was considered full SMBG acquisition; any beneficiary who scored <80% was defined as partial/no SMBG acquisition.
Outcome Measures
Primary outcome measures included relationship between full SMBG acquisition, partial/no SMBG acquisition, and survival probability over 4 years; change in percentage of beneficiaries with full and partial/no SMBG acquisition from 2009 through 2012; change in SMGB supply acquisition channel (retail or mail order) from 2010 through 2011; and impact of migration from full SMBG acquisition to partial/no SMBG acquisition on mortality, inpatient admissions, and associated costs from 2010 through 2011.
Data Source
The data sources used for our analyses were Medicare Beneficiary Annual Summary Files 2009–2010 and the Medicare Master Beneficiary Summary File: Base Segment, Chronic Conditions Segment, and the Cost and Utilization Segment 2011–2012 (BASF). BASF is used to identify Fee-for-Service beneficiaries. Fee-for-Service beneficiaries are defined here as those enrolled in Part A and Part B Medicare for at least 10 months in which Medicare was the primary payer and excludes any beneficiaries enrolled in Medicare Advantage, due to the lack of complete reimbursement data. The total reimbursement for inpatient stays for each beneficiary was captured from the BASF variables MEDREIMB_IP. Mortality was calculated from BASF variable BENE_DOD (date of death), and the number of unique hospitalizations was derived from BASF variable IPSTY.
Medicare durable medical equipment (DME) regional carrier (DMERC) File 2009–2013 was used to determine if a beneficiary ordered SMBG supplies. DME uses the Healthcare Common Procedure Coding System (HCPCS) A4253 to code for diabetes testing strips. If a beneficiary purchased diabetes testing strips, he/she would have an HCPCS A4253 in their DME record. The record would also contain the service date and the amount of SMBG testing supplies acquired. Each record for the purchase of diabetes testing strips also contained a modifier in which a “KL” code designated that the diabetes testing strips were acquired through mail order. The absence of the modifier noted a retail purchase.
We used the PROD_SRVC_ID (a reformatted version of the insulin’s national drug code) variable in Medicare Part D Event File 2009–2012 to determine if a beneficiary was on insulin therapy. We used the following PROD-SRVC_ID codes for insulin to identify the type of insulin obtained: 00088250033, 00088250052, 68115074610, 00002879959, 00002872559, 00002872501, 00002751559, 00002751001, 00002751659, 00002879859, 00002879359, 00002751201, 00002879759, 00002879459, 00002751101, 00002966001, 00002951501, 00002877059, 00002877001, 00002871501, 54868274600, 49999099310, 54569346700, 00002871759, 00002841501, 00002873001, 00002831501, 00002873059, 54569231800, 68115072905, 54569231900, 68115072810, 00002850101, 00002821501, 54569560500, 49999099410, 68115083910, 00088222052, 00088222033, 00088222060, 00169643910, 00169368712, 00169347718, 00169183711, 54868347400, 32849070801, 59060183702, 59060231704, 00169231721, 00169183717, 00169347418, 00169183411, 32849070601, 59060183402, 00169231421, 00169347318, 00169231321, 00169183311, 32849070701, 68115070905, 59060183302, 32849050081, 00169750111, 00169633910, 00169330312, 00169369619, 00169368512, and 00169368213.
Study Population
The study population comprised Medicare beneficiaries with a diagnosis of diabetes and a record of insulin treatment in 2009 (n = 529,627). This study population was separated into two groups, experimental and control, for analysis. The experimental group included all insulin-treated beneficiaries who resided in the nine CBP markets (TEST, n = 43,939) in 2009. The control group included all nontest market insulin-treated beneficiaries (NONTEST, n = 485,688). Among the types of insulin used, CMS records showed that 349,200 (65.9%) of beneficiaries were treated with short- or rapid-acting insulin (including premixed insulins) with or without long-acting or NPH insulin, whereas 180,427 (34.1%) were treated with long-acting or NPH insulin only.
Analysis
The TEST and NONTEST beneficiaries were subdivided: those who were full insulin acquisition and either full SMBG or partial/no SMBG acquisition (Table 1). A beneficiary was characterized as full or partial acquisition of insulin based upon the PDC model. PDC for insulin was calculated based on the fill dates and days of supply for each prescription filled in the Medicare Part D Event File. The numerator was the total number of days covered by the medication fills during the measurement period; the patient-level denominator was the number of days between the first fill and the end of the study period or death. Following Leslie’s time-array method (11), we calculated the PDC of insulin for each year from 2009 through 2012.
We then followed these clusters within the TEST and NONTEST groups year to year, from 2009 through 2012, to determine whether beneficiaries remained in one cluster or migrated to the other. This allowed us to identify any patterns in mortality, mortality rates, inpatient admissions, and costs. Of particular interest were changes that occurred between 2010 and 2011 when the CBP was implemented in the nine TEST markets. Analyses were performed to determine whether acquisition of insulin and SMBG supplies informed these results.
Differences between the TEST and NONTEST groups were compared and tested with χ2 tests. We performed survival analyses to examine the relationship between the two acquisition clusters in the total study population and within each study group: 1) those who maintained full SMBG acquisition during the whole study period and 2) those who maintained partial SMBG acquisition or not using SMBG (Table 1).
For survival probability, we followed 326,970 of the 529,627 beneficiaries for 4 years from 2009 through 2012. The remaining 202,657 were not included in our analysis through attrition due to early mortality or migration from Fee-for-Service to Medicare Advantage. Survival time was measured as days from the time the study began. A censoring variable was created to indicate the time of a death. We estimated the survivor function for each cluster from the residual survival times. The homogeneity tests were conducted to identify significant differences among the survival curves.
We used logistic regression models with outcome of SMBG supply acquisition for insulin-treated beneficiaries. The outcome event counts, including migration patterns, mortality rates, inpatient admissions, and medical costs, were compared. Because of the imbalance in number of beneficiaries in the TEST versus NONTEST populations, absolute percentages were used to describe beneficiary characteristics (e.g., partial/no SMBG), whereas relative percentages were used to describe changes in characteristics. Because we were looking at actual patient records, use of 95% CIs or SDs was not required.
It is noteworthy that the TEST cohort included a disproportionately higher percentage of Hispanic beneficiaries than the NONTEST cohort. Propensity score–matched analysis was performed to reduce selection bias due to imbalance in study covariates. We used logistic regression models with outcome of SMBG compliance for insulin-treated beneficiaries, adjusting for their baseline demographics and medical comorbidities. The beneficiaries in the partial/no SMBG group were matched to those in the full SMBG group in a 1:1 ratio based on the resultant propensity score probabilities. Matching was done using a generalized SAS macro for propensity score matching by Fraeman and Malley (12). The distribution of demographic factors and medical conditions at baseline was demonstrated (Table 1). The outcome event counts, including migration patterns, mortality rates, inpatient admissions, and medical costs, were compared after propensity score matching for new full SMBG and partial/no SMBG clusters. Because the CMS records did not include data about beneficiaries’ socioeconomic or educational status, a more refined analysis was not possible.
Results
Change in Full Versus Partial/No SMBG Acquisition
A higher percentage of TEST versus NONTEST beneficiaries showed full SMBG acquisition in 2009 (32.3% [n = 14,179 of 43,939] vs. 26.8% [n = 130,298 of 485,688]) and 2010 (32.2% [n = 10,864 of 33,790] vs. 27.6% [n = 104,939 of 380,904]). From 2010 to 2011, the percentage of beneficiaries with full SMBG acquisition decreased from 32.2% (n = 10,864 of 33,790) to 28.7% (n = 6,567 of 22,871; P < 0.0001) in TEST beneficiaries but increased from 27.5% (n = 104,939 of 380,904) to 28.2% (n = 81,092 of 287,957; P < 0.0001) in NONTEST beneficiaries. From 2010 to 2011, the percentage of beneficiaries with partial/no SMBG acquisition increased from 22.1% (n = 7,465 of 33,790) to 27.2% (n = 6,216 of 22,871; P < 0.0001) in the TEST cohort. The percentage of NONTEST beneficiaries with partial/no SMBG acquisition increased from 22.3% (n = 84,935 of 380,904) to 22.7% (65,329 of 287,957; P = 0.0002).
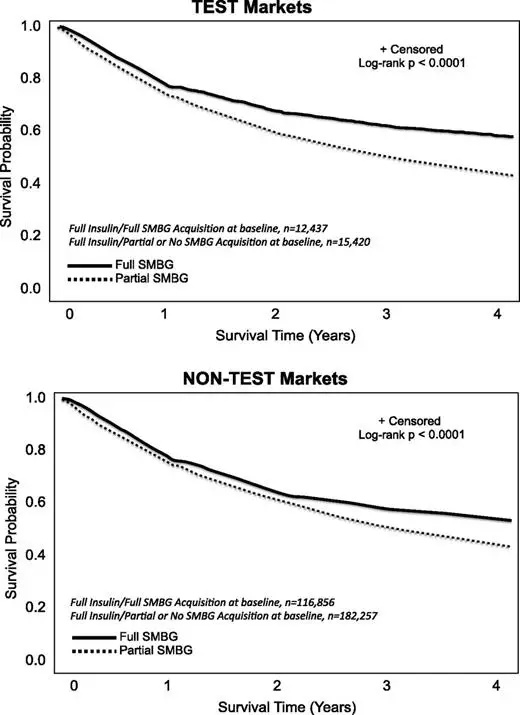
Survival Probability Associated With Full Versus Partial/No SMBG Acquisition
Four-year survival was negatively associated with partial/no SMBG acquisition or no SMBG record in both cohorts (P < 0.0001) (Fig. 1). In both study groups, mortality was higher among beneficiaries with full SMBG acquisition in 2010 who migrated to partial/no SMBG acquisition or no SMBG record in 2011 compared with maintaining full SMBG acquisition: TEST, 11.5 vs. 6.6%; NONTEST, 11.7 vs. 6.2%. However, mortality at 4 years was lower among beneficiaries with partial/no SMBG acquisition or no SMBG record in 2010 but migrated to full SMBG acquisition in 2011: TEST, 8.2%; NONTEST, 7.2%. Similar associations were seen in propensity score–matched analysis.
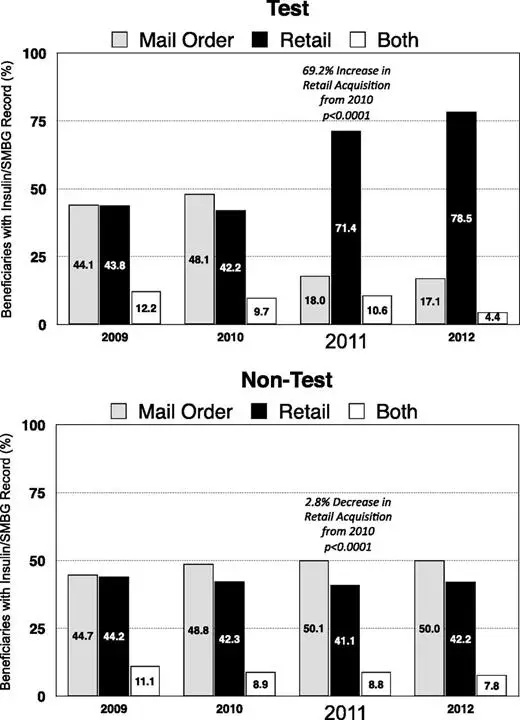
Change in SMBG Acquisition Channel
A notable shift in SMBG acquisition from the mail-order to retail channel was seen in the TEST cohort but not the NONTEST cohort (Fig. 2).
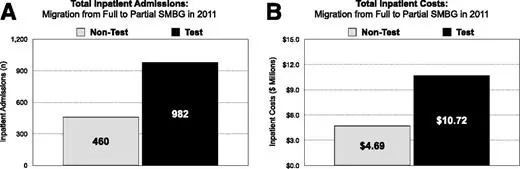
Impact of Migration From Full to Partial/No SMBG Acquisition
As presented in Table 2, propensity score–matched analysis showed that the percentage of beneficiaries who migrated from full SMBG in 2009 to partial/no SMBG in 2010 was similar in both the TEST and NONTEST groups. However, the percentage of TEST group beneficiaries who migrated from full SMBG in 2010 to partial/no SMBG in 2011 increased 58.1% (P < 0.0001), whereas the percentage of NONTEST beneficiaries who migrated from full to partial/no SMBG decreased 14.4% (P < 0.0001). Moreover, mortality was significantly higher among both TEST and NONTEST beneficiaries who migrated from full SMBG acquisition to partial/no SMBG acquisition. The disproportionate migration between groups was associated with 42 additional deaths within the TEST cohort, which was likely due to the increased number of beneficiaries who migrated from full to partial/no SMBG acquisition. Within the TEST market, migration from full SMBG to partial/no SMBG was associated with sex (female), ethnicity (black), and obstructive pulmonary disease and heart failure, whereas no significant associations between sex, ethnicity, or comorbidities were seen in the NONTEST cohort (Table 3). Mortality among TEST beneficiaries who migrated from full to partial/no SMBG in 2011 was significantly associated with chronic kidney disease, whereas atrial fibrillation was more predictive of mortality among NONTEST beneficiaries (Table 3).
More than twice as many inpatient hospital admissions were seen among TEST beneficiaries who migrated from full to partial/no SMBG acquisition compared with NONTEST beneficiaries (P < 0.0001) (Fig. 3A). Inpatient costs were also more than twice as high for TEST versus NONTEST beneficiaries who migrated to partial/no SMBG acquisition (P < 0.0001) (Fig. 3B).
Conclusions
Findings from our retrospective, longitudinal analysis of the CMS data set demonstrate that our hypothesis regarding the potential benefits of the CBP to Medicare beneficiaries was incorrect. The results indicate that the system implemented to reduce cost was associated with a disruption of acquisition of SMBG supplies as shown by the shift in SMBG acquisition channels (from mail order to retail) and significant increase in the percentage of beneficiaries in the TEST group versus NONTEST group who migrated from full SMBG to partial/no SMBG acquisition during the first year of CBP implementation. As discussed, sex (female) and ethnicity (black) were significantly associated with migration. Moreover, this migration was associated with an increase in mortality, an increase in inpatient admissions, and higher inpatient costs. Because full SMBG acquisition was strongly associated with better clinical outcomes compared with partial/no SMBG acquisition, these findings are particularly concerning, given the predominant use of short-acting insulin and rapid-acting insulin analogs by Medicare beneficiaries, who are at significantly greater risk for hypoglycemia than younger individuals with insulin-treated diabetes (3,4).
We are troubled that CMS failed to detect these “unintended” consequences and, instead, reported that the pilot program was a success (10) but without comment on the impacts we discovered in our analyses. In this regard, it is noteworthy that our findings are somewhat supported by a separate report by the Government Accountability Office to Congress (13). The Government Accountability Office reported that the monitoring methods used by CMS in assessing the impact of competitive bidding did not show directly whether beneficiaries received the DME needed on time, or whether health outcomes were caused by problems accessing the CBP-covered DME (13), thereby calling the CMS findings into question.
A key strength of our study design was use of longitudinal analysis. This approach allowed us to measure the occurrence of change in SMBG acquisition behaviors and subsequent outcomes at the individual level, providing the opportunity to observe individual patterns of change (14). When the goal of monitoring patient safety is to identify and assess the causal effect of certain treatments or interventions (e.g., CBP) on the outcome, longitudinal studies are preferred over nonlongitudinal ones in which the temporal order of treatment and outcome may be unclear (15), as seen in the CBP reporting. By comparing changes in health behaviors (e.g., acquisition of diabetic testing supplies) and attributable risks (e.g., mortality, hospitalizations, and costs), the impact of exposure to CBP can be estimated (16). Use of the propensity score analysis allowed us to identify an equivalent control group (17), thereby facilitating a true “apples-to-apples” comparison between beneficiaries who were affected by CBP and those not affected.
Several limitations of our analysis are noteworthy. Because the CMS data only provided information about SMBG supply acquisition by beneficiaries, it was not possible to link outcomes with actual utilization of SMBG. Another limitation is the lack of data provided by CMS regarding the causes of hospitalization. Moreover, the CMS records provided no information regarding the socioeconomic or educational characteristics of the beneficiaries. These data would have facilitated more refined propensity score matching and allowed us to determine if these factors confounded our comparisons between the TEST and NONTEST beneficiaries. Nevertheless, use of propensity score matching confirmed that changes in SMBG acquisition did occur and that the subsequent increases in mortality, hospitalizations, and hospitalization costs were likely related to implementation of the CBP within the TEST markets.
Specific causes of disruption and migration to partial/no SMBG acquisition could not be determined from the CMS data; however, several potential reasons can be suggested, including loss of previous supplier, difficulty in securing a new supplier (13,18), and violations of the Medicare Improvement Patient Protection Act (14), which prohibits suppliers from switching products from one brand to another (13). Monitoring supplier compliance with the antiswitching provisions is impossible because all Medicare claims for diabetes test strips are billed using one HCPCS code (A4253) regardless of brand. This lack of transparency creates a situation in which clinicians may be unaware of the SMBG systems their patients are using due to “switching” from branded to low-cost products, causing a “disconnect” between clinicians and patients, which increases the potential for inadequate patient training, resulting in more testing inaccuracies (19). This, in turn, could discourage SMBG use at prescribed levels. Additionally, because the program may have made it difficult for many beneficiaries to continue using the SMBG systems they were familiar with, it is possible that these beneficiaries reduced or discontinued their SMBG due to lack of training and/or lack of confidence in the accuracy of their new SMBG system (20).
Although the unintended consequences of CBP implementation in the TEST markets are alarming, the potential impact of the nationwide implementation of the program raises even greater concerns. When CMS implemented the national launch of the CBP for SMBG supplies in July 2013, reimbursement for test strips was reduced from approximately $35.00 to $10.41 per bottle of 50 strips when acquired through both mail-order and retail channels. This reduction may dissuade many pharmacies (especially independent pharmacies) from providing SMBG supplies to Medicare beneficiaries, which could further impact testing frequency among all Medicare beneficiaries. In a 2013 survey of more than 300 community pharmacists, 92% of respondents reported that the sharp reduction in payment for diabetes test strips would force them to leave the program (21).
It is not known whether this potential disruption of access has already impacted patient adherence to their testing regimens; however, the significant reduction in reimbursement for test strips will likely increase migration from compliance to noncompliance to a higher level than seen in the TEST markets as more beneficiaries become unable to obtain their current brand of SMBG supplies from retail channels. Moreover, because the CBP requires suppliers to resubmit bids every 3 years, the disruption seen in the TEST markets could be perpetual unless CMS initiates more effective monitoring protocols that safeguard beneficiaries.
In human clinical trials, investigators have an ethical obligation to monitor the safety of study participants and terminate the study immediately whenever risk to patients is detected (22), as was done in the ACCORD trial, which was stopped prematurely because of higher mortality in the intensive treatment arm compared with that in the standard treatment arm (23). Given the prospective approach taken in implementing competitive bidding, CMS should be held to the same safety monitoring standards as other clinical trials. As such, based on our findings, policy makers should consider suspending the CBP until CMS can demonstrate its ability to effectively monitor the effects of the program and ensure that Medicare beneficiaries, a population that is most vulnerable to both the acute and chronic complications of diabetes, are protected from harm.
Article Information
Duality of Interest. The National Minority Quality Forum, a nonprofit organization, received support for the study from Abbott; Acelity L.P., Inc.; LifeScan, Inc. (part of Johnson & Johnson Diabetes Solutions Companies); Roche Diabetes Care; US Healthcare Supply LLC; and US MED. R.A.V. is an employee of Medtronic. C.G.P. has received consulting fees from Animas Corporation; CeQur SA; Dexcom, Inc.; Roche Diabetes Care; and Sanofi. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. G.A.P. and L.X. developed the study protocol, contributed to data analysis, and wrote the manuscript. G.N.-B., F.Z., J.A.D., and R.A.V. contributed to data analysis. C.G.P. and D.G.M. contributed to data analysis and wrote the manuscript. All authors reviewed the manuscript and accept responsibility for the contents of this report. G.A.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Portions of the study findings were presented as posters at the American Association of Clinical Endocrinologists 24th Annual Scientific and Clinical Congress, Nashville, TN, 13–17 May 2015, and the 75th Scientific Sessions of the American Diabetes Association, Boston, MA, 5–7 June 2015.
